Ultrasound-released nanoparticles may help diabetics avoid the needle
November 25, 2013
[+]
A new nanotechnology-based technique for regulating blood sugar in
diabetics could give patients the ability to release insulin painlessly
using a small ultrasound device, allowing them to go days between
injections — rather than using needles to give themselves multiple
insulin injections each day.
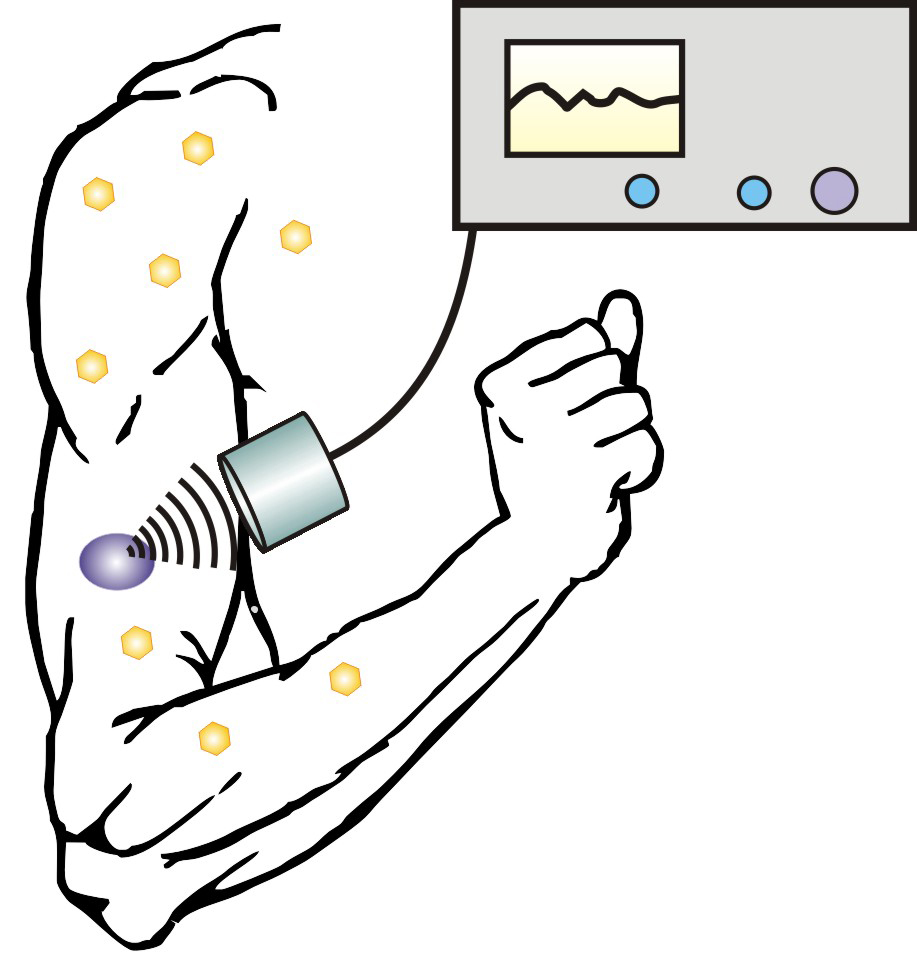
New
technique allows diabetics to control insulin release with an
injectable nano-network and portable ultrasound device (credit: NCSU)
A patient who has type 1 or advanced type 2 diabetes needs additional insulin, a hormone that transports glucose — or blood sugar — from the bloodstream into the body’s cells.
These diabetes patients must inject insulin as needed to ensure their blood sugar levels are in the “normal” range. However, these injections can be painful.
The new technique — developed by Dr. Zhen Gu, an assistant professor in the joint biomedical engineering program at NC State and UNC-Chapel Hill, and other researchers at North Carolina State University and the University of North Carolina at Chapel Hill — may offer a solution.
[+]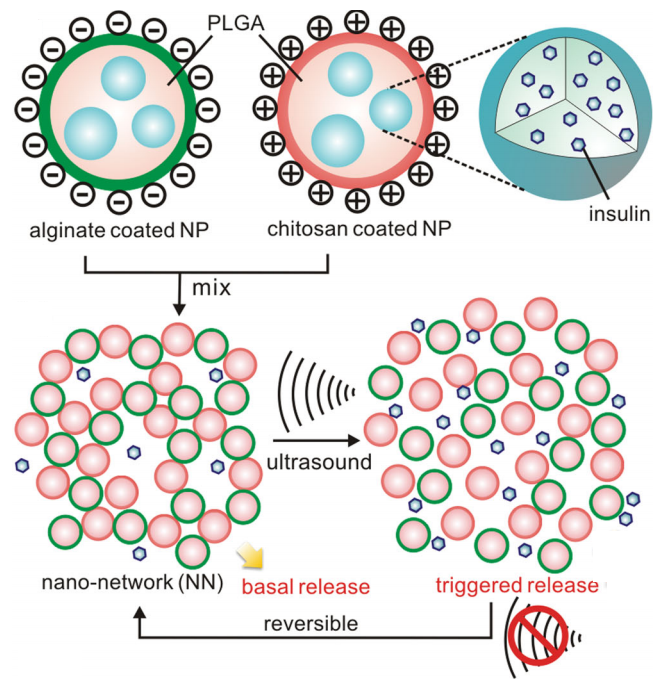
How the insulin delivery system works
Insulin delivery system (credit: Di et al./Advanced Healthcare Materials)
1. Biocompatible and biodegradable nanoparticles are injected into a patient’s skin. The nanoparticles are made out of poly(lactic-co-glycolic) acid (PLGA) and are filled with insulin.
(Each of the PLGA nanoparticles is given either a positively charged coating made of chitosan (a biocompatible material normally found in shrimp shells), or a negatively charged coating made of alginate (a biocompatible material normally found in seaweed). When the solution of coated nanoparticles is mixed together, the positively and negatively charged coatings are attracted to each other by electrostatic force to form a “nano-network.”)
2. Once injected into the subcutaneous layer of the skin, that nano-network holds the nanoparticles together and prevents them from dispersing throughout the body.
3. The injected insulin begins to diffuse from the coated PLGA nanoparticles, which are also porous. But most of the insulin doesn’t stray far — it is suspended in a de facto reservoir in the subcutaneous layer of the skin by the electrostatic force of the nano-network. This essentially creates a dose of insulin that is simply waiting to be delivered into the bloodstream.
4. The patient can use a small, hand-held device to apply focused ultrasound waves to the site of the nano-network, painlessly releasing the insulin from its de facto reservoir into the bloodstream.
When the ultrasound is removed, the electrostatic force reasserts itself and pulls the nanoparticles in the nano-network back together. The nanoparticles then diffuse more insulin, refilling the reservoir.
5. When the insulin runs out, a new nano-network has to be injected.
Ultrasonic waves generate microscope gas bubbles
The researchers believe the technique works because the ultrasound waves excite microscopic gas bubbles in the tissue, temporarily disrupting the nano-network in the subcutaneous layer of the skin. That disruption pushes the nanoparticles apart, relaxing the electrostatic force being exerted on the insulin in the reservoir.
This allows the insulin to begin entering the bloodstream — a process hastened by the effect of the ultrasound waves pushing on the insulin.
“We know this technique works, and we think this is how it works, but we are still trying to determine the precise details,” says Dr. Yun Jing, an assistant professor of mechanical engineering at NC State and co-corresponding author of the paper.
“We’ve done proof-of-concept testing in laboratory mice with type 1 diabetes,” Gu says. “We found that this technique achieves a quick release of insulin into the bloodstream, and that the nano-networks contain enough insulin to regulate blood glucose levels for up to 10 days.”
“The system may be available commercially in a few years, but we first need to perform large animal studies and clinical trials, Gu told KurzweilAI. Gu is also a senior author of a paper on the research.
“Compared to the traditional insulin delivery method (needle/syringe based), our method is non-invasive, painless, with quick response. and can last a long time (one injection can last over a week, or even longer),” he said.
This work was supported by NC TraCS, NIH’s Clinical and Translational Science Awards at UNC-CH.
Abstract of Advanced Healthcare Materials paper
An on demand, non-invasive and portable insulin delivery method that can achieve pulsatile insulin release and effective regulation of blood glucose is highly desirable for type 1 and advanced type 2 diabetes administration. We report that integration of an injectable nano-network with a focused ultrasound system (FUS) can remotely regulate insulin release both in vitro and in vivo. Serving as a synthetic insulin reservoir, the nano-network consisting of adhesive poly(lactic-co-glycolic acid) nanoparticles significantly promoted insulin release upon intermittent FUS triggers. Remarkably, a maximum of 80-fold increase in the insulin release rate was observed when the nano-network was exposed to the irradiation of ultrasound for 30 sec. In vivo studies validated that this method provided repeatable and spatiotemporal regulation of blood glucose levels in Type 1 diabetic mice. A single subcutaneous injection of the nano-network with intermittent FUS administration facilitated reduction of the blood glucose levels for up to 10 days.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington