First ‘mini-kidney’ structures from human stem cells generated by Salk scientists
Findings may lead to much-needed therapies for kidney disease
November 19, 2013
[+]
A team of researchers led by scientists at the Salk Institute for Biological Studies
has generated three-dimensional kidney structures from human stem cells
for the first time, opening new avenues for studying development and
diseases of the kidneys and discovery of new drugs that target human
kidney cells.
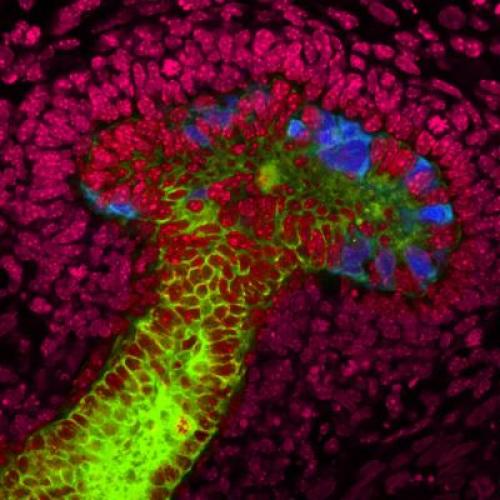
For
the first time, Salk scientists have grown human stem cells into
early-stage ureteric buds (kidney structures responsible for reabsorbing
water after toxins have been filtered out). In the laboratory, the
scientists used mouse embryonic kidney cells (seen here in red) to coax
the human stem cells to grow into the nascent mushroom-shaped buds (blue
and green). Their discovery is a major step in developing regenerative
techniques for growing replacement human kidneys. (Credit: Salk
Institute for Biological Studies)
The findings were reported November 17, 2013 in Nature Cell Biology.
Diseases affecting the kidneys represent a major and unsolved health issue worldwide. The kidneys rarely recover function once they are damaged by disease, highlighting the urgent need for better knowledge of kidney development and physiology.
First kidney-like 3D structures
Scientists had created precursors of kidney cells using stem cells as recently as this past summer, but the Salk team was the first to coax human stem cells into forming three-dimensional cellular structures similar to those found in our kidneys.
“Attempts to differentiate human stem cells into renal cells have had limited success,” says senior study author Juan Carlos Izpisua Belmonte, a professor in Salk’s Gene Expression Laboratory and holder of the Roger Guillemin Chair. “We have developed a simple and efficient method that allows for the differentiation of human stem cells into well-organized 3D structures of the ureteric bud (UB), which later develops into the collecting duct system.”
The Salk findings demonstrate for the first time that pluripotent stem cells (PSCs) — cells capable of differentiating into the many cells and tissue types that make up the body — can be made to develop into cells similar to those found in the ureteric bud, an early developmental structure of the kidneys, and then be further differentiated into three-dimensional structures in organ cultures.
UB cells form the early stages of the human urinary and reproductive organs during development and later develop into a conduit for urine drainage from the kidneys. The scientists accomplished this with both human embryonic stem cells and induced pluripotent stem cells (iPSCs) — human cells from the skin that have been reprogrammed into their pluripotent state.
Growth factors
After generating iPSCs that demonstrated pluripotent properties and were able to differentiate into mesoderm, a germ cell layer from which the kidneys develop, the researchers made use of growth factors known to be essential during the natural development of our kidneys for the culturing of both iPSCs and embryonic stem cells.
The combination of signals from these growth factors, molecules that guide the differentiation of stem cells into specific tissues, was sufficient to commit the cells toward progenitors that exhibit clear characteristics of renal cells in only four days.
The researchers then guided these cells to further differentiated into organ structures similar to those found in the ureteric bud by culturing them with kidney cells from mice. This demonstrated that the mouse cells were able to provide the appropriate developmental cues to allow human stem cells to form three-dimensional structures of the kidney.
Test on a patient with kidney disease
In addition, Izpisua Belmonte’s team tested their protocol on iPSCs from a patient clinically diagnosed with polycystic kidney disease (PKD), a genetic disorder characterized by multiple, fluid-filled cysts that can lead to decreased kidney function and kidney failure. They found that their methodology could produce kidney structures from patient-derived iPSCs.
Because of the many clinical manifestations of the disease, neither gene- nor antibody-based therapies are realistic approaches for treating PKD. The Salk team’s technique might help circumvent this obstacle and provide a reliable platform for pharmaceutical companies and other investigators studying drug-based therapeutics for PKD and other kidney diseases.
“Our differentiation strategies represent the cornerstone of disease modeling and drug discovery studies,” says lead study author Ignacio Sancho-Martinez, a research associate in Izpisua Belmonte’s laboratory. “Our observations will help guide future studies on the precise cellular implications that PKD might play in the context of kidney development. ”
Researchers at the Center of Regenerative Medicine in Barcelona, Spain and Josep Maria Campistol of the Hospital Clinic of Barcelona were also involved in the study.
The work was supported by the California Institute for Regenerative Medicine, the Nomis Foundation, Fundacion Cellex, the G. Harold and Leila Y. Mathers Charitable Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, Fundació La Marató de TV3, CIBER BBN and ISCIII-TERCEL-MINECO.
Abstract of Nature Cell Biology paper
Diseases affecting the kidney constitute a major health issue worldwide. Their incidence and poor prognosis affirm the urgent need for the development of new therapeutic strategies. Recently, differentiation of pluripotent cells to somatic lineages has emerged as a promising approach for disease modelling and cell transplantation. Unfortunately, differentiation of pluripotent cells into renal lineages has demonstrated limited success. Here we report on the differentiation of human pluripotent cells into ureteric-bud-committed renal progenitor-like cells. The generated cells demonstrated rapid and specific expression of renal progenitor markers on 4-day exposure to defined media conditions. Further maturation into ureteric bud structures was accomplished on establishment of a three-dimensional culture system in which differentiated human cells assembled and integrated alongside murine cells for the formation of chimeric ureteric buds. Altogether, our results provide a new platform for the study of kidney diseases and lineage commitment, and open new avenues for the future application of regenerative strategies in the clinic.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington