Engineered glowing worms detect neural effects of drugs
November 18, 2013
[+]
A research team at Worcester Polytechnic Institute (WPI)
and The Rockefeller University in New York has developed a novel system
to image brain activity in multiple awake and unconstrained worms.
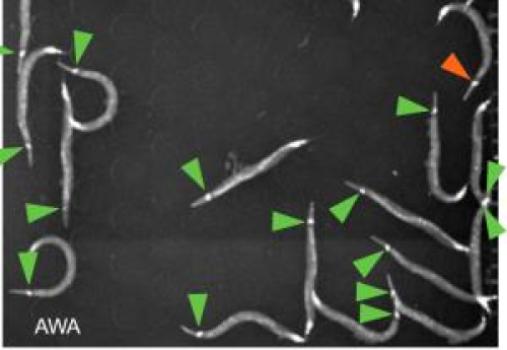
Neurons in the worms (marked by arrows) glow as the animals sense attractive odors (credit: Worcester Polytechnic Institute)
The technology makes it possible to study the genetics and neural circuitry associated with animal behavior, but it can also be used as a high-throughput screening tool for drug development targeting autism, anxiety, depression, schizophrenia, and other brain disorders.
The team details their technology and early results in the paper “High-throughput imaging of neuronal activity in Caenorhabditis elegans,” published by the journal Proceedings of the National Academy of Sciences (open access).
“One of our major objectives is to understand the neural signals that direct behavior — how sensory information is processed through a network of neurons leading to specific decisions and responses,” said Dirk Albrecht, PhD, assistant professor of biomedical engineering at WPI and senior author of the paper.
To study neuronal activity, Albrecht’s lab uses the tiny worm Caenorhabditis elegans (C. elegans), a nematode found in many environments around the world. A typical adult C. elegans is just 1 millimeter long and has 969 cells, of which 302 are neurons.
Despite its small size, the worm is a complex organism able to do all of the things animals must do to survive. It can move, eat, mate, and process environmental cues that help it forage for food or react to threats. As a bonus for researchers, C.elegans is transparent. By using various imaging technologies, including optical microscopes, one can literally see into the worm and watch physiological processes in real time.
Numerous studies have been done by “worm labs” around the world exploring various neurological processes in C. elegans. These have typically been done using one worm at a time, with the animal’s body fixed in place on a slide.
[+]
Engineering glowing worms for automated testing 
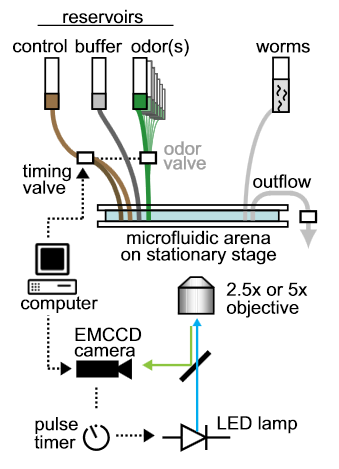
Schematic of the automated microscope. Video acquisition and fl uidic valves for chemical stimulation are computer-controlled;
fluorescence illumination is pulsed via digital signals synchronized with each camera frame. No motorized stage or focus mechanisms are needed with low-magnication objectives and glass-mounted microfluidic arenas.
fluorescence illumination is pulsed via digital signals synchronized with each camera frame. No motorized stage or focus mechanisms are needed with low-magnication objectives and glass-mounted microfluidic arenas.
In their new paper, Albrecht’s team details how they imaged, recorded, and analyzed specific neurons in multiple animals as they wormed their way around a custom-designed microfluidic array, called an arena, where they were exposed to favorable or hostile sensory cues.
Specifically, the team engineered a strain of worms with neurons near the head that would glow when they sensed food odors. In experiments involving up to 23 worms at a time, Albrecht’s team infused pulses of attractive or repulsive odors into the arena and watched how the worms reacted.
In general, the worms moved towards the positive odors and away from the negative odors, but the behaviors did not always follow this pattern. “We were able to show that the sensory neurons responded to the odors similarly in all the animals, but their behavioral responses differed significantly,” Albrecht said. “These animals are genetically identical, and they were raised together in the same environment, so where do their different choices come from?”
In addition to watching the head neurons light up as they picked up odor cues, the new system can trace signaling through “interneurons.” These are pathways that connect external sensors to the rest of the network (the “worm brain”) and send signals to muscle cells that adjust the worm’s movement based on the cues.
Testing drug effects on neural networks
Numerous brain disorders in people are believed to arise when neural networks malfunction. In some cases the malfunction is dramatic overreaction to a routine stimulus, while in others it is a lack of appropriate reactions to important cues.
Since C. elegans and humans share many of the same genes, discovering genetic causes for differing neuronal responses in worms could be applicable to human physiology. Experimental compounds designed to modulate the action of nerve cells and neuronal networks could be tested first on worms using Albrecht’s new system. The compounds would be infused in the worm arena, along with other stimuli, and the reaction of the worms’ nervous systems could be imaged and analyzed.
“The basis of our work is to combine biomedical engineering and neuroscience to answer some of these fundamental questions and hopefully gain insight that would be beneficial for understanding and eventually treating human disorders,” Albrecht said.
Abstract of Proceedings of the National Academy of Sciences paper
Neuronal responses to sensory inputs can vary based on genotype, development, experience, or stochastic factors. Existing neuronal recording techniques examine a single animal at a time, limiting understanding of the variability and range of potential responses. To scale up neuronal recordings, we here describe a system for simultaneous wide-field imaging of neuronal calcium activity from at least 20 Caenorhabditis elegans animals under precise microfluidic chemical stimulation. This increased experimental throughput was used to perform a systematic characterization of chemosensory neuron responses to multiple odors, odor concentrations, and temporal patterns, as well as responses to pharmacological manipulation. The system allowed recordings from sensory neurons and interneurons in freely moving animals, whose neuronal responses could be correlated with behavior. Wide-field imaging provides a tool for comprehensive circuit analysis with elevated throughput in C. elegans.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington