The secret of longevity for the world’s longest-living rodent: better protein creation
October 3, 2013
[+]
Better-constructed proteins could explain why naked mole rats live long lives — about 30 years — and stay healthy until the very end, resisting cancer, say University of Rochester biologists Vera Gorbunova and Andrei Seluanov.
Naked
mole rats are small, hairless, subterranean rodents native to eastern
Africa (credit: Adam Fenster/University of Rochester)
Their work focuses on naked mole rat ribosomes, which assemble amino acids into proteins. Ribosomes are composed of ribosomal RNA (rRNA) molecules and proteins.
When the ribosome connects amino acids together to create a protein, a mistake is occasionally introduced when an incorrect amino acid is inserted. But the researchers found that the proteins made by naked mole rat cells are up to 40 times less likely to contain such mistakes than the proteins made by mouse cells.
Gorbunova and Seluanov discovered a possible reason: the naked mole rat’s rRNA is unique.
Unique rRNA pattern
[+]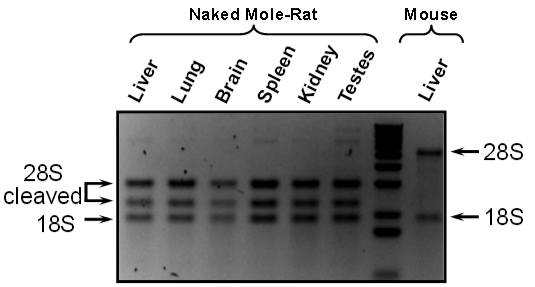
After applying a dye to a sample, they studied it under ultraviolet
light. They found three dark bands — representing concentrations of
different rRNA molecules — not the two bands that are characteristic of
all other animals, suggesting that there is a “hidden break” in the
naked mole rat rRNA.
Naked
mole rat rRNA in this NMR image shows an unusual pattern with cleaved
28S pattern: 3 bands, instead of 2 with mouse (credit: Jorge Azpurua et
al./PNAS)
When its rRNA strands cleave (split) at two specific locations, instead of floating off on their own, two remaining pieces from each strand stay close to each other. They act as a scaffold on which ribosomal proteins are assembled to create a functional ribosome, preventing aberrations. “This is important because proteins with no aberrations help the body to function more efficiently,” said Seluanov.
The next step for the biologists is to split mouse rRNA in the same way to see if it would lead to improved protein creation.
The two biologists hope their work will eventually result in pharmaceutical treatments that modulate protein synthesis in humans, though any medical solution is a long way off, they say.
Abstract for PNAS paper
The naked mole-rat (Heterocephalus glaber) is a subterranean eusocial rodent with a markedly long lifespan and resistance to tumorigenesis. Multiple data implicate modulation of protein translation in longevity. Here we report that 28S ribosomal RNA (rRNA) of the naked mole-rat is processed into two smaller fragments of unequal size. The two breakpoints are located in the 28S rRNA divergent region 6 and excise a fragment of 263 nt. The excised fragment is unique to the naked mole-rat rRNA and does not show homology to other genomic regions. Because this hidden break site could alter ribosome structure, we investigated whether translation rate and amino acid incorporation fidelity were altered. We report that naked mole-rat fibroblasts have significantly increased translational fidelity despite having comparable translation rates with mouse fibroblasts. Although we cannot directly test whether the unique 28S rRNA structure contributes to the increased fidelity of translation, we speculate that it may change the folding or dynamics of the large ribosomal subunit, altering the rate of GTP hydrolysis and/or interaction of the large subunit with tRNA during accommodation, thus affecting the fidelity of protein synthesis. In summary, our results show that naked mole-rat cells produce fewer aberrant proteins, supporting the hypothesis that the more stable proteome of the naked mole-rat contributes to its longevity.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington