Hydrogel implant enables light-based communication with cells inside the body
October 25, 2013
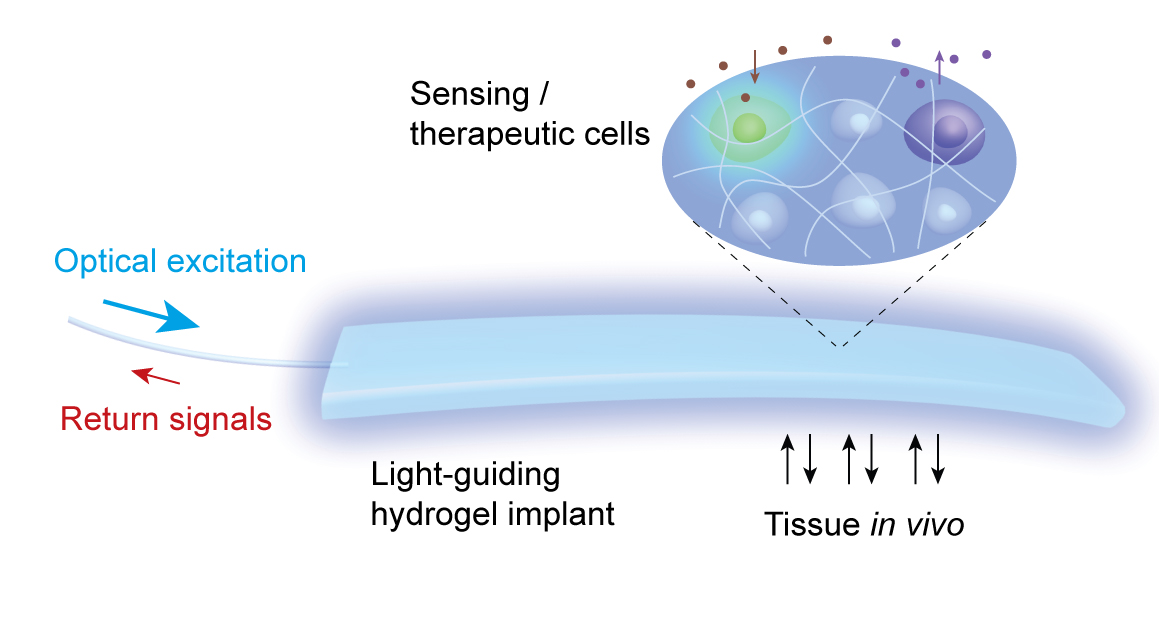
Light
passing through an optical fiber (left) can either carry in a signal
that stimulates the activity of cells embedded in the hydrogel implant
(for treatment) or bring back a signal generated by cells responding to
something in their environment (credit: Harvard Bio-Optics Lab/Wellman
Center for Photomedicine, Mass. General Hospital)
The use of light to communicate with cells has previously been restricted by its limited ability to pass through tissues, especially the skin.
Called a light-guiding hydrogel, the implant is constructed from a polymer-based scaffolding capable of supporting living cells and contains cells genetically engineered either to carry out a specific activity in response to light or to emit light in response to a particular metabolic signal. An optical fiber connects the implant to either an external light source or a light detector.
The first system’s implants contained cells genetically engineered to express light-emitting green fluorescent protein (GFP) upon contact with a toxin. After confirming in vitro the hydrogels’ response to nanoparticles containing the toxic metal cadmium, the researchers implanted the hydrogels beneath the skin of three groups of mice.
Treatment with blue light
To investigate a possible therapeutic application for the system, the investigators used a hydrogel implant containing cells that respond to blue light by producing glucagon-like peptide-1 (GLP-1), a protein playing an essential role in glucose metabolism.
After the implants were placed under the skin of mice with diabetes, the blue light signal was delivered for 12 hours. A day and a half later — 48 hours after the implant — the animals that received the light signal had double the level of GLP-1 in their blood, along with significantly better results in a glucose tolerance test, than did implanted mice not treated with light.
“This work combines several existing technologies well known in their respective fields — such as drug delivery, genetic engineering, biomaterial science, and photonics — to build a new implant system that enables the delivery of photomedicine deep in the body,” says ”Wellman investigator Seok Hyun (Andy) Yun, PhD, senior author of the study and an associate professor of Dermatology at Harvard Medical School and director of the Harvard Bio-Optics Lab.
“This is the first time anyone has shown the ability to talk optically — by means of light — with cells deep within the body, both to sense the presence of a toxin and to deliver a cell-based therapy.”
Clinical use and future studies
“Clinical translation will take time and effort because there are issues in using transgenic cells and light-guiding efficiency for human-scale implant,” Myunghwan Choi, first author of the Nature Photonics article, explained to KurzweilAI.
“We are planning to investigate how changing the shape and structure of the hydrogel can improve the implant’s light-guiding properties, ways to improve the production and delivery of a therapeutic protein, how the immune system would react to long-term implantation, and ways to deliver or detect the light signal that would not require passing a fiber through the skin.
“The main innovation of the research is the interdisciplinary nature of the work. We combined existing technologies well known in their respective fields — such as drug delivery, genetic engineering, biomaterial science, and photonics — to build a new implant system that enables the delivery of photomedicine deep in the body.The research is broadly applicable for diagnosis and therapy using light.”
Korea Advanced Institute of Science and Technology was also involved in the study. Support for the study includes grants from the National Institutes of Health, National Science Foundation, and Department of Defense.
Abstract of Nature Photonics paper
Polymer hydrogels are widely used as cell scaffolds for biomedical applications. Although the biochemical and biophysical properties of hydrogels have been investigated extensively, little attention has been paid to their potential photonic functionalities. Here, we report cell-integrated polyethylene glycol-based hydrogels for in vivo optical-sensing and therapy applications. Hydrogel patches containing cells were implanted in awake, freely moving mice for several days and shown to offer long-term transparency, biocompatibility, cell viability and light-guiding properties (loss of <1 cm="" db="" nbsp="" sup="">−1
). Using optogenetic, glucagon-like peptide-1 secreting cells, we conducted light-controlled therapy using the hydrogel in a mouse model with diabetes and obtained improved glucose homeostasis. Furthermore, real-time optical readout of encapsulated heat-shock-protein-coupled fluorescent reporter cells made it possible to measure the nanotoxicity of cadmium-based bare and shelled quantum dots (CdTe; CdSe/ZnS) in vivo.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington