A super antioxidant based on material used in vehicle catalytic converters
Could help treat traumatic brain injury, cardiac arrest, and
Alzheimer’s patients, guard against radiation-induced side effects
suffered by cancer patients, perhaps even slow the effects of aging
October 22, 2013
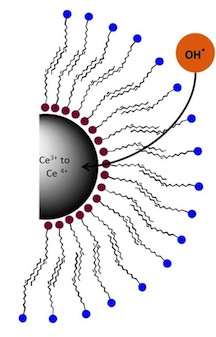
Oleylamine
(red dots) and oleac acid (blue) layers serve to protect a cerium oxide
nanosphere that catalyzes reactive oxygen species by absorbing and
turning them into less-harmful molecules. The finding could help treat
injuries, guard against radiation-induced side effects of cancer therapy
and protect astronauts from space radiation. (Credit: Colvin Group/Rice
University)
Rice chemist Vicki Colvin led a team that created small, uniform spheres of cerium oxide and gave them a thin coating of fatty oleic acid to make them biocompatible.
The researchers say their discovery has the potential to help treat traumatic brain injury, cardiac arrest and Alzheimer’s patients and could guard against radiation-induced side effects suffered by cancer patients.
Their nanoparticles also have potential to protect astronauts from long-term exposure to radiation in space and perhaps even slow the effects of aging, they reported.
Extreme antioxidant
Cerium oxide nanocrystals can absorb and release oxygen ions — a chemical reaction known as reduction oxidation (redox). It’s the same process that allows catalytic converters in cars to absorb and eliminate pollutants.
The particles made at Rice are small enough to be injected into the bloodstream when organs need protection from oxidation, particularly after traumatic injuries, when damaging reactive oxygen species (ROS) increase dramatically.
The cerium particles go to work immediately, absorbing ROS free radicals, and they continue to work over time as the particles revert to their initial state, a process that remains a mystery, Colvin said. The oxygen species released in the process “won’t be super reactive.” .
How it works
Cerium oxide, a form of the rare earth metal cerium, remains relatively stable as it cycles between cerium oxide III and IV. In the first state, the nanoparticles have gaps in their surface that absorb oxygen ions like a sponge. When cerium oxide III is mixed with free radicals, it catalyzes a reaction that effectively defangs the ROS by capturing oxygen atoms and turning into cerium oxide IV. Cerium oxide IV particles slowly release their captured oxygen and revert to cerium oxide III, and can break down free radicals again and again.
Colvin said the nanoparticles’ tiny size makes them effective scavengers of oxygen. “The smaller the particles, the more surface area they have available to capture free radicals. A gram of these nanoparticles can have the surface area of a football field, and that provides a lot of space to absorb oxygen.”
None of the cerium oxide particles made before Rice tackled the problem were stable enough to be used in biological settings, she said. “We created uniform particles whose surfaces are really well-defined, and we found a water-free production method to maximize the surface gaps available for oxygen scavenging.”
Colvin said it’s relatively simple to add a polymer coating to the 3.8-nanometer spheres. The coating is thin enough to let oxygen pass through to the particle, but robust enough to protect it through many cycles of ROS absorption.
A better, safer radioprotectant for cancer patients
In testing with hydrogen peroxide, a strong oxidizing agent, the researchers found their most effective cerium oxide III nanoparticles performed nine times better than a common antioxidant, Trolox, at first exposure, and held up well through 20 redox cycles.
“The next logical step for us is to do some passive targeting,” Colvin said. “For that, we plan to attach antibodies to the surface of the nanoparticles so they will be attracted to particular cell types, and we will evaluate these modified particles in more realistic biological settings.”
Colvin is most excited by the potential to help cancer patients undergoing radiation therapy. “Existing radioprotectants have to be given in incredibly high doses,” she said. “They have their own side effects, and there are not a lot of great options.”
She said a self-renewing antioxidant that can stay in place to protect organs would have clear benefits over toxic radioprotectants that must be eliminated from the body before they damage good tissue.
The Center for Biological and Environmental Nanotechnology, the Advanced Energy Consortium, the Welch Foundation, and the National Science Foundation supported the research.
Abstract of ACS Nano paper
This work examines the effect of nanocrystal diameter and surface coating on the reactivity of cerium oxide nanocrystals with H2O2 both in chemical solutions and in cells. Monodisperse nanocrystals were formed in organic solvents from the decomposition of cerium precursors, and subsequently phase transferred into water using amphiphiles as nanoparticle coatings. Quantitative analysis of the antioxidant capacity of CeO2-x using gas chromatography and a luminol test revealed that two moles of H2O2 reacted with every mole of cerium (III), suggesting that the reaction proceeds via a Fenton-type mechanism. Smaller diameter nanocrystals containing more cerium (III) were found to be more reactive towards H2O2. Additionally, the presence of a surface coating did not preclude the reaction between the nanocrystal surface cerium (III) and hydrogen peroxide. Taken together, the most reactive nanoparticles were the smallest (e.g. 3.8 nm diameter) with the thinnest surface coating (e.g. oleic acid). Moreover, a benchmark test of their antioxidant capacity revealed these materials were nine times more reactive than commercial antioxidants such as Trolox. A unique feature of these antioxidant nanocrystals is that they can be applied multiple times: over weeks, cerium (IV) rich particles slowly return to their starting cerium (III) content. In nearly all cases, the particles remain colloidally stable (e.g. non-aggregated) and could be applied multiple times as anti-oxidants. These chemical properties were also observed in cell culture, where the materials were able to reduce oxidative stress in human dermal fibroblasts exposed to H2O2 with efficiency comparable to their solution phase reactivity. These data suggest that organic coatings on cerium oxide nanocrystals do not limit the antioxidant behavior of the nanocrystals, and that their redox cycling behavior can be preserved even when stabilized.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington