Converting fat cells from liposection to liver cells in nine days — a regenerative medicine breakthrough
New method would replace costly, highly invasive liver
transplantation, could be available for clinical testing in two to three
years
October 23, 2013
[+]
A fast, efficient way to turn cells extracted from routine
liposuction into liver cells — a feat with huge potential for
regenerative medicine — has been developed by Stanford University School of Medicine scientists
A
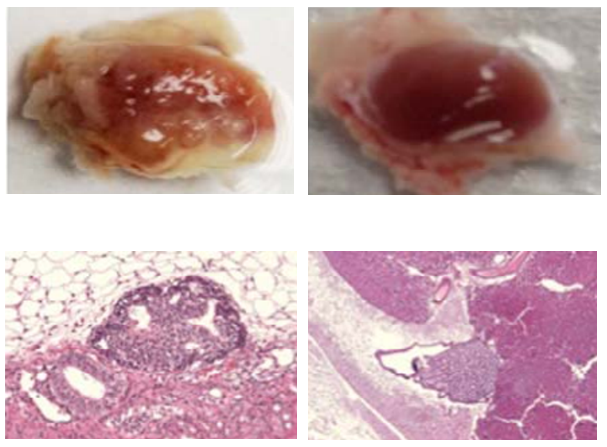
new method of creating liver cells does not form tumors in
immunocompromised mice. Three weeks after induced pluripotent stem cells
(left, magnification below) were implanted in test tissue, palpable
tumors were formed in the area of implantation. In contrast, no tumors
were detected 2 months after the same number of the new spherical
liver-like cells were implanted (right, magnification below). (Credit:
Dan Xu et al./Cell Transplantation)
The scientists performed their experiments in mice, but the adipose (fat) stem cells they used came from human liposuction and actually became human, liver-like cells that flourished inside the mice’s bodies.
This method is distinct from those producing liver cells from embryonic stem cells or induced pluripotent stem cells (iPS). Although embryonic and iPS stem cells are pluripotent — they can, in principle, differentiate into every cell type — they carry a palpable risk of forming tumors.
However, the cells produced using this new technique, which involves no intermediate pluripotent phase, show no signs of being tumorogenic (creating tumors).
All aspects of the new fat-to-liver technique are adaptable for human use, said Gary Peltz, MD, PhD, Stanford professor of anesthesia and the study’s senior author. Creating iPS cells requires introducing foreign and potentially carcinogenic genes. But adipose stem cells merely have to be harvested from fat tissue.
The process takes nine days from start to finish — fast enough to regenerate liver tissue in acute liver poisoning victims, who would otherwise die within a few weeks, barring liver transplantation.
How they did it
Liver cells are not something an adipose stem cell normally wants to turn into, Peltz said.
The Stanford team knew it was possible, though. Another way of converting liposuction-derived adipose stem cells to liver-like cells (called i-Heps, for induced hepatocytes) had been developed in 2006 by Japanese researchers. But that method, which relies on chemical stimulation, requires 30 days or longer and is inefficient; it could not produce enough material for liver reconstitution. (Working with iPS cells takes even longer; they must first be generated from adult cells before they can be converted to i-Heps.)
Using a different technique — Peltz refers to it as “spherical culture” — he and his associates were able to achieve the conversion within nine days with an efficiency of 37 percent, as opposed to the vastly lower yield obtained with the prior method (12 percent) or using iPS cells. (Peltz said improvements since the study’s publication now enable yields exceeding 50 percent within seven to eight days.)
Dan Xu, PhD, a postdoctoral scholar and the study’s lead author, adapted the spherical culture methodology from early embryonic-stem-cell literature. Instead of growing on flat surfaces in a laboratory dish, the harvested adipose stem cells are cultured in a liquid suspension in which they form spheroids. “This seems to make them happier,” Peltz said.
When they had enough cells, the investigators tested them by injecting them into immune-deficient laboratory mice that accept human grafts. These mice were bioengineered in 2007, in a collaboration between Peltz’s lab and study co-author Toshihiko Nishimura, MD, PhD, and other scientists at the Tokyo-based Central Institute for Experimental Animals. Only the livers of these mice contained an extra gene that would convert the antiviral compound gancyclovir into a potent toxin. When these mice were treated with gancyclovir, their liver cells died off quickly.
At this point the investigators injected 5 million i-Heps into the mice’s livers. To do that — no mean feat, as these tiny organs weigh a scant 1.8 grams — they used an ultrasound-guided injection procedure that is routinely employed in gastroenterological clinics for biopsies.
Four weeks later, the investigators examined the mice’s blood and found the presence of a protein (human serum albumin) that is only produced by human liver cells and was shown to be an accurate proxy for the number of new human liver cells in these experimental mice’s livers.
The mice’s blood had substantial human serum albumin levels, which nearly tripled in the following four weeks. These blood levels correspond with the repopulation of roughly 10–20 percent of the mice’s pre-destroyed livers by new human liver tissue. (Past studies have shown only miniscule human serum albumin production, at best, in mice given similar amounts of chemically induced i-Heps.)
Blood tests also revealed that the mice’s new liver tissue was discharging its waste-filtration responsibility. Examination of the livers themselves showed that the transplanted cells had integrated into the liver, expressed surface markers unique to mature human hepatocytes and produced multi-cell structures required for human bile duct formation. Other tests indicated that the spherically cultured i-Heps resembled natural human hepatocytes more closely than did i-Heps produced from iPS cells.
Importantly, two months after injection of i-Heps produced by spherical culture, there was no evidence of tumor formation. But mice in which IPS-cell-originated i-Heps were introduced developed multiple tumors, which could be felt through the body surface within three weeks.
At 1,500 grams, a healthy human liver is more than 800 times the size of a mouse’s and contains about 200 billion cells. “To be successful, we must regenerate about half of the damaged liver’s original cell count,” said Peltz. With spherical culture, he said, close to a billion injectable iHeps can be produced from 1 liter of liposuction aspirate, readily obtained from a single liposuction procedure. The cell replication that takes place after injection expands that number further, to over 100 billion i-Heps.
Ready for clinical trial in two to three years
That could be enough to substitute for a human liver transplant, Peltz said. Stanford’s Office of Technology Licensing has filed a patent on the use of spherical culture for hepatocyte induction. Peltz’s group is optimizing the culture and injection techniques, talking to the U.S. Food and Drug Administration, and gearing up for safety tests on large animals.
“We believe our method will be transferable to the clinic,” Peltz said. “And because the new liver tissue is derived from a person’s own cells, we do not expect that immunosuppressants will be needed.”
“We hope to begin clinical testing in 2015/16,” Peltz told KurzweilAI. This new method “would represent an advance in the treatment of end stage liver failure, which would be achieved using a 21st century regenerative medicine procedure,” he said. “Among the various different approaches being utilized to accomplish this, this method represents the one that is scalable for human use, can be performed using procedures that translate to the clinic, and appears to be the method with the highest safety.”
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases.
Facts about liver damage and transplants
The liver is the body’s chemistry set. It builds complex biomolecules we need, and it filters and breaks down waste products and toxic substances that might otherwise accumulate to dangerous levels. Unlike most other organs, a healthy liver can regenerate itself to a significant extent. But this capacity cannot overcome acute liver poisoning or damage from chronic alcoholism or viral hepatitis.
Acute liver failure from acetaminophen alone takes about 500 lives annually and accounts for close to 60,000 emergency-room visits and more than 25,000 hospitalizations annually. Other environmental toxins, including poisonous mushrooms, contribute still more cases.
Some 6,300 liver transplants are performed annually in the United States, with another 16,000 patients on the waiting list. Every year, more than 1,400 people die before a suitable liver can be found for them. While it can save lives, liver transplantation is complicated, risky and, even when successful, fraught with aftereffects. Typically, the recipient is consigned to a lifetime of taking immunosuppressant drugs to prevent organ rejection.
————————————————————————————————————————————————————————————-
Abstract of Cell Transplantation paper
We developed a novel method for differentiating adipocyte-derived stem cells (ASCs) into hepatocyte-like cells (iHeps). ASCs are cultured as spherical cellular aggregates, and are then induced by culture in chemically defined media for a short time period to differentiate into spherical-culture iHeps (SCi-Heps). SCi-Heps have many of the in vitro functional properties of mature hepatocytes, and they can stably reconstitute functioning human liver in vivo in a murine model system, and implantation studies demonstrate that SCi-Heps have a very low malignant potential. All human liver regenerative procedures, including ultrasound-guided direct liver implantation, are scalable and appropriate for human clinical use. These methods can be used to achieve the major promise of regenerative medicine; it may now be possible to regenerate human liver using autologous stem cells obtained from a readily accessible tissue
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington