New 3D method used to grow miniature pancreas model
Could help in the fight against diabetes
October 17, 2013
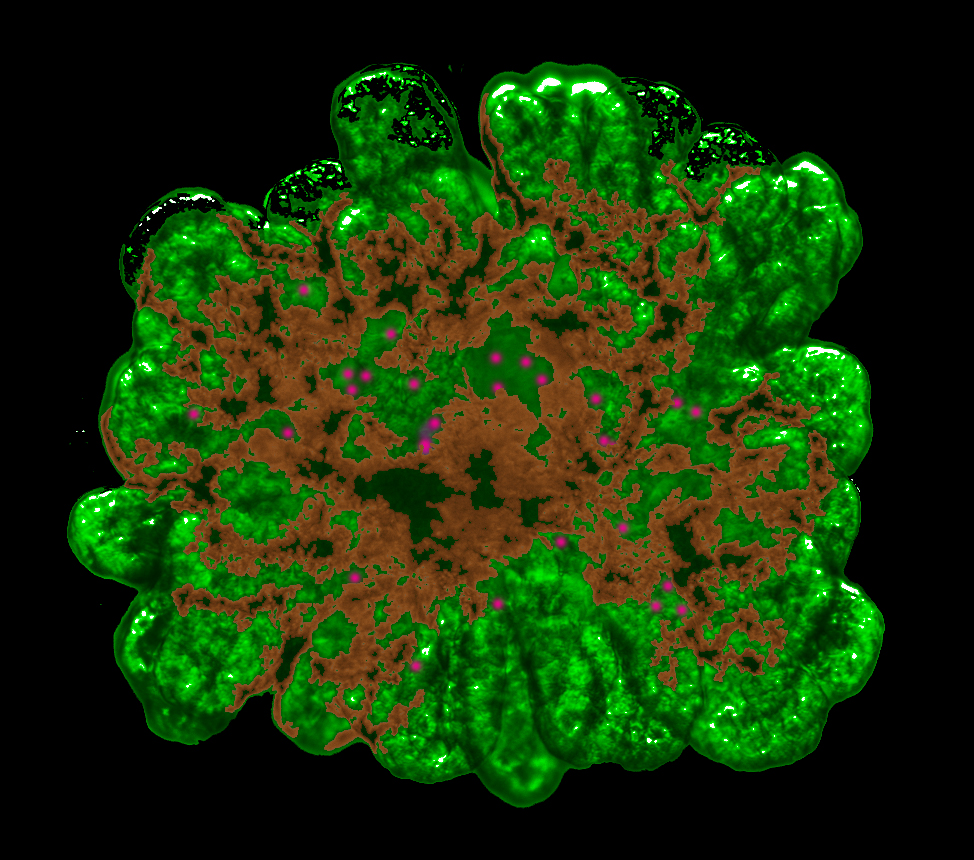
The
new method allows the cell material from mice to grow vividly in
picturesque tree-like structures (credit: University of Copenhagen)
The goal is to use this model to help in the fight against diabetes.
Using cell material from mice, Professor Anne Grapin-Botton and her team at the Danish Stem Cell Centre developed a three-dimensional culture method, allowing pancreatic cells to grow vividly in picturesque tree-like structures.
“The new method allows the cell material to take a three-dimensional shape, enabling them to multiply more freely. It’s like a plant where you use effective fertilizer; think of the laboratory like a garden and the scientist being the gardener,” says Grapin-Botton.
In the future, the researchers hope to produce a miniature human pancreas from human stem cells. These human miniature organs would be valuable as models to test new drugs fast and effective — without the use of animal models.
Social cells
The cells do not thrive and develop if they are alone; a minimum of four pancreatic cells close together is required for subsequent organoid development.
“We found that the cells of the pancreas develop better in a gel in three dimensions than when they are attached and flattened at the bottom of a culture plate. Under optimal conditions, the initial clusters of a few cells have proliferated into 40,000 cells within a week. The clusters transform into cells that make either digestive enzymes or hormones like insulin, and they self-organize into branched pancreatic organoids that are amazingly similar to the pancreas,” adds Anne Grapin-Botton.
Using this system, the scientists discovered that the cells are sensitive to their physical environment, such as the stiffness of the gel and to contact with other cells.
Pancreas and diabetes connection
An effective cellular therapy for diabetes is dependent on the production of sufficient quantities of functional beta cells, the scientists say. Recent studies have enabled the production of pancreatic precursors but efforts to expand these cells and differentiate them into insulin-producing beta-cells have proved a challenge.
“We think this is an important step towards the production of cells for diabetes therapy, both to produce mini-organs for drug testing and to for insulin-producing cells as spare parts. We show that the pancreatic cells care not only about how you feed them but need to be grown in the right physical environment. We are now trying to adapt this method to human stem cells,” adds Grapin-Botton.
“The innovation in our research is that it’s the only model that recapitulates both the shape (morphogenesis) and the differentiation of the pancreas, starting from a few cells, Grapin-Botton told KurzweilAI. “Future adaptations of this culture system to human ES and iPS cells would provide a model of pancreas development for drug testing. And starting from iPS cells from patients, the system could be used as a disease model.
“Such adaptations are anticipated to take one or two years of development. It could be used for the production of beta cells but the efficiency of differentiation would need to be increased to get more pure populations.”
The research results were just published in the scientific journal Development (open access for one month)
Abstract of Development paper
In the context of a cellular therapy for diabetes, methods for pancreatic progenitor expansion and subsequent differentiation into insulin-producing beta cells would be extremely valuable. Here we establish three-dimensional culture conditions in Matrigel that enable the efficient expansion of dissociated mouse embryonic pancreatic progenitors. By manipulating the medium composition we generate either hollow spheres, which are mainly composed of pancreatic progenitors, or complex organoids that spontaneously undergo pancreatic morphogenesis and differentiation. The in vitro maintenance and expansion of pancreatic progenitors require active Notch and FGF signaling, thus recapitulating in vivo niche signaling interactions. Our experiments reveal new aspects of pancreas development, such as a community effect by which small groups of cells better maintain progenitor properties and expand more efficiently than isolated cells, as well as the requirement for three-dimensionality. Finally, growth conditions in chemically defined biomaterials pave the way for testing the biophysical and biochemical properties of the niche that sustains pancreatic progenitors.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington