Compound blocks neurodegeneration in mice
October 30, 2013
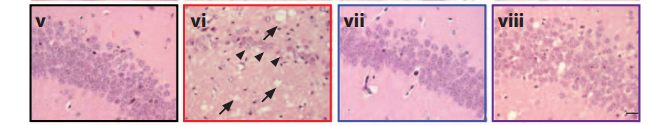
[+]
An orally administered compound that prevents neurodegeneration in mice has been developed by researchers at the Medical Research Council (MRC) Toxicology Unit at the University of Leicester.
GSK2606414
drug prevents spongiosis, gliosis, and neurodegeneration (vi) in
hippocampus of prion-infected mice at 7 weeks (vii) and 9 weeks (viii),
compared to normal tissue (v) (credit: Julie A. Moreno et al., Science Translational Medicine)
The team had found previously (Nature) that the build up of misfolded proteins in the brains of mice with prion disease over-activates a natural defense mechanism in cells, which switches off the production of new proteins.
This mechanism would normally switch back on again, but in these mice, the continued buildup of misshapen protein keeps the switch turned off. This is the trigger point leading to brain cell death, since the key proteins essential for nerve cell survival stop being made.
Originally, the team injected a protein that blocked the “off” switch of the pathway into a small region of the brain, and by doing this were able to restore protein production, and halt the neurodegeneration. The brain cells were protected, and protein levels and synaptic transmission (the way in which brain cells signal to each other) were restored allowing the mice to live longer. This led the scientists to predict that compounds able to block this pathway would also protect brain cells.
In the new study, published in Science Translational Medicine, the researchers gave by mouth a drug-like compound against the pathway to prion infected mice, hoping to block the off-switch in the same way. The compound (originally been developed by GlaxoSmithKline for a different purpose) was able to enter the brain from the bloodstream and halt the disease, throughout the whole brain.
However, this compound, despite protecting the brain, also produced weight loss in the mice and mild diabetes, due to damage to the pancreas.*
The researchers studied mice with prion disease because these mouse models currently provide the best animal representation of human neurodegenerative disorders in which the buildup of misshapen proteins is linked with brain cell death. These include Alzheimer’s and Parkinson’s as well as prion diseases. Another paper in Nature Neuroscience last month highlighted this pathway as a potential therapeutic target in treating Alzheimer’s.
“We’re still a long way from a usable drug for humans — this compound had serious side effects,” said University of Leicester Professor Giovanna Mallucci. But the fact that we have established that this pathway can be manipulated to protect against brain cell loss first with genetic tools and now with a compound, means that developing drug treatments targeting this pathway for prion and other neurodegenerative diseases is now a real possibility.”
“Misshapen proteins in prion diseases and other human neurodegenerative disorders, such as Alzheimer’s and Parkinson’s, also over-activate this fundamental pathway controlling protein synthesis in the brains of patients,” said Professor Hugh Perry, chair of the MRC’s Neuroscience and Mental Health Board.
“Despite the toxicity of the compound used, this study indicates that, in mice at least, we now have proof-of-principle of a therapeutic pathway that can be targeted. This might eventually aid the development of drugs to treat people suffering from dementias and other devastating neurodegenerative diseases.”
Abstract of Science Translational Medicine paper
During prion disease, an increase in misfolded prion protein (PrP) generated by prion replication leads to sustained overactivation of the branch of the unfolded protein response (UPR) that controls the initiation of protein synthesis. This results in persistent repression of translation, resulting in the loss of critical proteins that leads to synaptic failure and neuronal death. We have previously reported that localized genetic manipulation of this pathway rescues shutdown of translation and prevents neurodegeneration in a mouse model of prion disease, suggesting that pharmacological inhibition of this pathway might be of therapeutic benefit. We show that oral treatment with a specific inhibitor of the kinase PERK (protein kinase RNA–like endoplasmic reticulum kinase), a key mediator of this UPR pathway, prevented UPR-mediated translational repression and abrogated development of clinical prion disease in mice, with neuroprotection observed throughout the mouse brain. This was the case for animals treated both at the preclinical stage and also later in disease when behavioral signs had emerged. Critically, the compound acts downstream and independently of the primary pathogenic process of prion replication and is effective despite continuing accumulation of misfolded PrP. These data suggest that PERK, and other members of this pathway, may be new therapeutic targets for developing drugs against prion disease or other neurodegenerative diseases where the UPR has been implicated.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington