Method for mass production of graphene-based field-effect transistors (FETs) developed
December 20, 2013
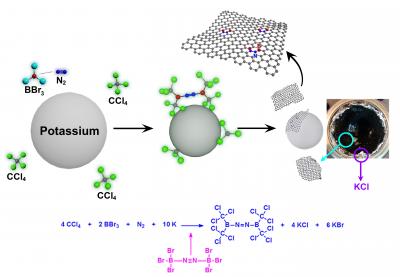
Schematic
representation of the formation of BCN-graphene via solvothermal
reaction between carbon tetrachloride (CCl4), boron tribromide (BBr3),
and nitrogen (N2) in the presence of potassium (K). Photograph is of the
autoclave after the reaction, showing the formation of BCN-graphene
(black) and potassium halide (KCl and KBr, white). (Credit: UNIST)
The design creates boron/nitrogen co-doped graphene nanoplatelets (BCN-graphene) via a simple solvothermal reaction of BBr3/CCl4/N2 in the presence of potassium.
Various methods of making graphene-based FETs have been exploited, including doping graphene, tailoring graphene like a nanoribbon, and using boron nitride as a support, the researchers said.
Among the methods of controlling the bandgap* of graphene, doping methods show the most promise in terms of industrial-scale feasibility, they suggest.
Researchers have previously tried to add boron to graphene to open its bandgap to achieve semiconductor performance, without success, because the atomic size of boron, 85 pm (atomic radius) is larger than that of carbon (77 pm).
Now, the UNIST researcher team, led by Prof. Jong-Beom Baek, has found that boron/nitrogen co-doping is only feasible when carbon tetrachloride (CCl4 ) is treated with boron tribromide (BBr3 ) and nitrogen (N2) gas, which at 70 pm is a bit smaller than carbon and boron.
Pairing two nitrogen atoms and two boron atoms can compensate for the atomic size mismatch, so boron and nitrogen pairs can be easily introduced into the graphitic network, the researchers say. The resultant BCN-graphene generates a bandgap appropriate for FETs.
“Although the performance of the FET is not in the range of commercial silicon-based semiconductors, this initiative work should be the proof of a new concept and a great leap forward for studying graphene with bandgap opening,” said Baek. “Now, the remaining challenge is fine-tuning a bandgap to improve the on/off current ratio for real device applications.”
This work will be published in Angewandte Chemie International Edition as a VIP (“very important paper”).
The research work was funded by the National Research Foundation (NRF) of Korea and the U.S. Air Force Office of Scientific Research through the Asian Office of Aerospace R&D (AFOSR-AOARD).
* A bandgap is the energy required to allow an electron to move freely within a solid material — a major factor determining the electrical conductivity of a solid. Substances with large band gaps are generally insulators; conductors (such as native graphene) either have very small band gaps or none. Those with intermediate bandgaps are semiconductors.
Abstract of Angewandte Chemie International Edition paper
Boron/nitrogen co-doped graphene (BCN graphene) is directly synthesized from a reaction between CCl4/BBr3/N2 in the presence of potassium. The reaction of CCl4 with either BBr3 or N2 alone leads to a marginally doped graphene. On the other hand, there is a synergistic effect when CCl4 is reacted with BBr3 and N2 together to yield BCN graphene. The resultant BCN graphene displays good dispersion stability in N-methyl-2-pyrrolidone, allowing for the fabrication of a field-effect transistor by solution casting, which displays an on/off ratio of 10.7 with an optical band gap of 3.3 eV. Considering the scalability and solution processability, BCN graphene has a high potential for many practical applications.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington