Forcing cancer cells to shape-shift stops them from migrating, Mayo Clinic researchers find
December 12, 2013
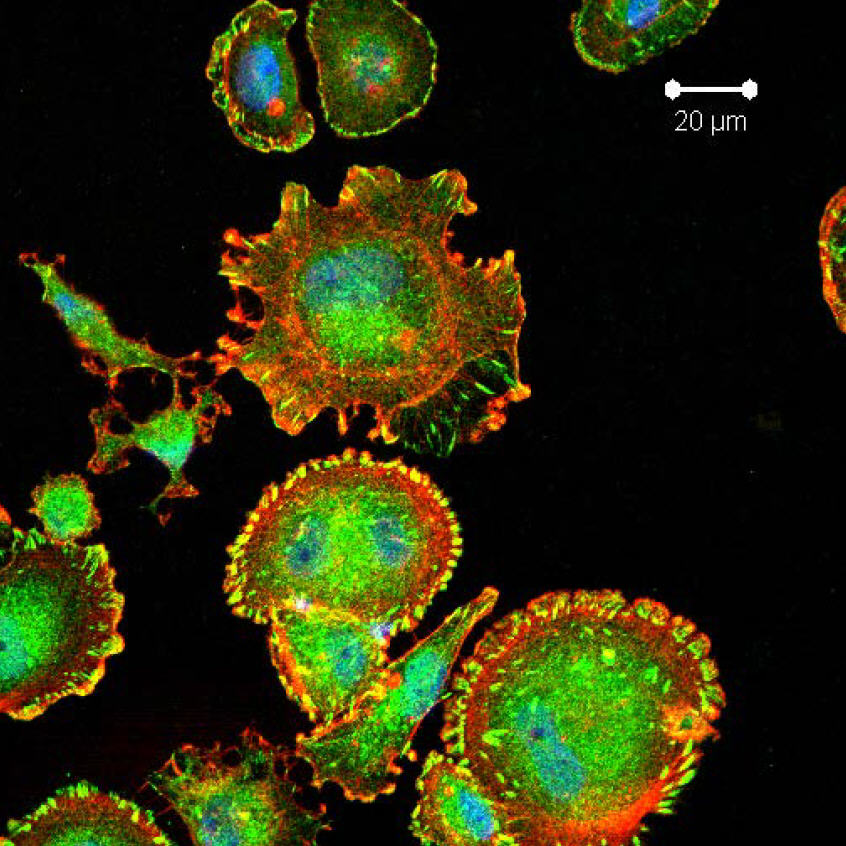
Glioblastoma tumor cells that are depleted of Syx and assume the fried egg phenotype (credit: Mayo Clinic)
“We are starting to understand mechanistically how cancer cells move and migrate, which gives us opportunities to manipulate these cells, alter their shape, and stop their spread,” says the study’s lead investigator, Panos Z. Anastasiadis, Ph.D., chair of the Department of Cancer Biology at Mayo Clinic in Florida.
“It is the spread — the metastasis — of cancer that is largely responsible for the death of cancer patients, so stopping these cells from migrating could potentially provide a treatment that saves lives,” he says.
The study was conducted using tumor material from breast and brain (glioblastoma) cancer. Both of these tumors are generally lethal when they spread — breast to other organs, and brain cancer as it crawls throughout the brain.
The researchers found that a protein called Syx is key to determining how tumor cells migrate. When researchers removed Syx from the cancer cells, they lost their polarity — their leading and trailing edges — and morphed into a “fried egg” shape. “They are now unable to sense direction, so they are not going anywhere,” Dr. Anastasiadis says.
Abstract of Molecular and Cellular Biology paper
The role of RhoA in promoting directed cell migration has been complicated by studies showing that it is activated both in the front and the rear of migrating cells. We report here that the RhoA-specific guanine nucleotide exchange factor Syx is required for the polarity of actively migrating brain and breast tumor cells. This function of Syx is mediated by the selective activation of the RhoA downstream effector Dia1, the subsequent reorganization of microtubules, and the downregulation of focal adhesions and actin stress fibers. The data argue that directed cell migration requires the precise spatiotemporal regulation of Dia1 and ROCK activities in the cell. The recruitment of Syx to the cell membrane and the subsequent selective activation of Dia1 signaling, coupled with the suppression of ROCK and activation of cofilin-mediated actin reorganization, plays a key role in establishing cell polarity during directed cell migration.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington