Drug-carrying nanoparticles delivered orally to replace injections
May have a major impact on the treatment of many diseases by
enabling drugs currently limited by low bioavailability to be
efficiently delivered though oral administration
December 2, 2013
The new nanoparticles are coated with antibodies that act as a key to unlock receptors found on the surfaces of cells that line the intestine, allowing the nanoparticles to break through the intestinal walls and enter the bloodstream.
This type of drug delivery could be especially useful in developing new treatments for conditions such as high cholesterol and arthritis. Patients with those diseases would be much more likely to take pills regularly than to make frequent visits to a doctor’s office to receive nanoparticle injections, say the researchers.
“If you were a patient and you had a choice, there’s just no question: patients would always prefer drugs they can take orally,” says Robert Langer, the David H. Koch Institute Professor at MIT, a member of MIT’s Koch Institute for Integrative Cancer Research, and an author of the Science Translational Medicine paper.
Several types of nanoparticles carrying chemotherapy drugs or short interfering RNA, which can turn off selected genes, are now in clinical trials to treat cancer and other diseases. These particles exploit the fact that tumors and other diseased tissues are surrounded by leaky blood vessels. After the particles are intravenously injected into patients, they seep through those leaky vessels and release their payload at the tumor site.
But for these nanoparticles to be taken orally, they need to be able to get through the intestinal lining, which is made of a layer of epithelial cells that join together to form impenetrable barriers called tight junctions.
Bypassing the intestinal lining
To build nanoparticles that can selectively break through the barrier, the researchers took advantage of previous work that revealed how babies absorb antibodies from their mothers’ milk, boosting their own immune defenses. Those antibodies grab onto a cell surface receptor called the FcRN, granting them access through the cells of the intestinal lining into adjacent blood vessels.
[+]
After the particles are ingested, the Fc proteins grab on to the FcRN
in the intestinal lining and gain entry, bringing the entire
nanoparticle along with them.
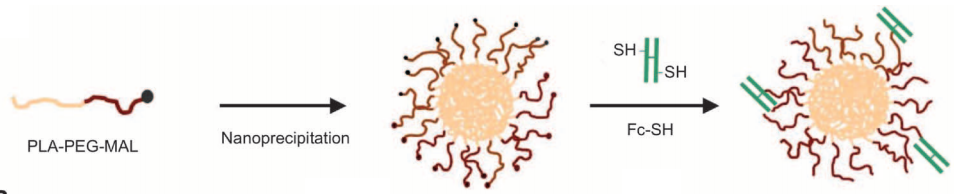
Schematic
of nanoparticle assembly: nanoparticles (left) consist of a PLA core
(yellow) for drug encapsulation and a PEG surface for stability and to
block phagocytes (center). They can carry a large drug payload, such as
insulin, in their core. They are then coated with Fc proteins — the part
of the antibody that binds to the FcRN receptor, which is also found in
adult intestinal cells — and thiol groups (SH). (Credit: E. M. Pridgen
et al./Science Translational Medicine)
[+]
In this study, the researchers demonstrated oral delivery of insulin
in mice. Nanoparticles coated with Fc proteins reached the bloodstream
11-fold more efficiently than equivalent nanoparticles without the
coating. Furthermore, the amount of insulin delivered was large enough
to lower the mice’s blood sugar levels.
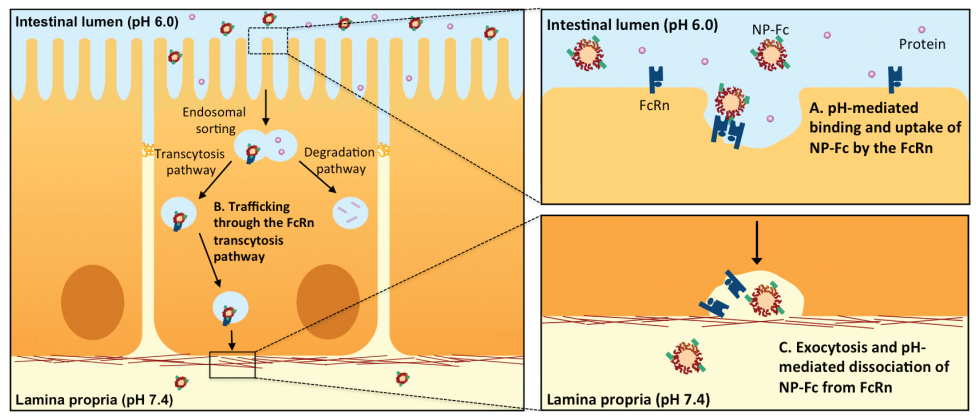
Penetrating
the intestinal lining: NP-Fc nanoparticles (top) penetrate the
intestinal lining down to the lamina propria (bottom), where the neutral
pH causes the release of the drug-bearing nanoparticles, which can then
diffuse through the lamina propria and enter systemic circulation.
(Credit: E. M. Pridgen et al./Science Translational Medicine)
Bl0od-brain barrier next
The researchers now hope to apply the same principles to designing nanoparticles that can cross other barriers, such as the blood-brain barrier, which prevents many drugs from reaching the brain.
They are also working on optimizing drug release from the nanoparticles in preparation for further animal tests, either with insulin or other drugs.
The research was funded by a Koch-Prostate Cancer Foundation Award in Nanotherapeutics; the National Cancer Institute Center of Cancer Nanotechnology Excellence at MIT-Harvard; a National Heart, Lung, and Blood Institute Program of Excellence in Nanotechnology Award; and the National Institute of Biomedical Imaging and Bioengineering.
Abstract of Science Translational Medicine paper
Nanoparticles are poised to have a tremendous impact on the treatment of many diseases, but their broad application is limited because currently they can only be administered by parenteral methods. Oral administration of nanoparticles is preferred but remains a challenge because transport across the intestinal epithelium is limited. We show that nanoparticles targeted to the neonatal Fc receptor (FcRn), which mediates the transport of immunoglobulin G antibodies across epithelial barriers, are efficiently transported across the intestinal epithelium using both in vitro and in vivo models. In mice, orally administered FcRn-targeted nanoparticles crossed the intestinal epithelium and reached systemic circulation with a mean absorption efficiency of 13.7%*hour compared with only 1.2%*hour for nontargeted nanoparticles. In addition, targeted nanoparticles containing insulin as a model nanoparticle-based therapy for diabetes were orally administered at a clinically relevant insulin dose of 1.1 U/kg and elicited a prolonged hypoglycemic response in wild-type mice. This effect was abolished in FcRn knockout mice, indicating that the enhanced nanoparticle transport was specifically due to FcRn. FcRn-targeted nanoparticles may have a major impact on the treatment of many diseases by enabling drugs currently limited by low bioavailability to be efficiently delivered though oral administration.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington