An electronic diagnostic pill for detecting early-stage gastric cancer
December 9, 2013
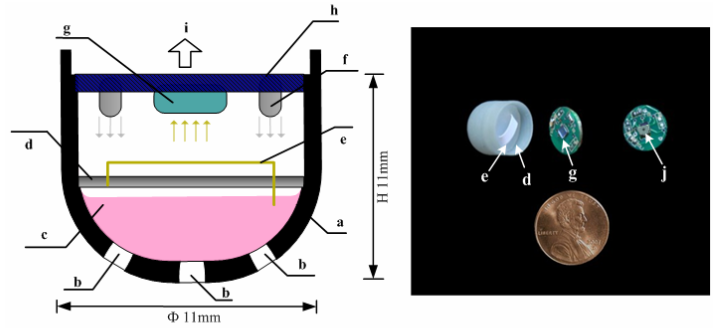
[+]
Researchers at Chongqing University in China have adapted capsule
endoscopy to allow for detecting tiny quantities of “occult” blood for
screening of early-stage gastric cancer.
Gastric
Occult Blood (GOB) system. Occult blood test paper (e), LED (f),
imaging sensor (g), printed circuit board (h), video signal (i), lens
(j). Batteries not shown. (Credit: Hongying Liu et al./International
Journal of Biomedical Engineering and Technology)
The data is automatically transmitted to an external monitoring device in real time for diagnosis by a physician.
The non-invasive Gastric Occult Blood (GOB) capsule, which carries inside a detector, power supply, and wireless transmitter, is encased in non-toxic, acid-safe polycarbonate. The device has a detection limit of 6 micrograms per liter of fluid. Laboratory tests have demonstrated its simplicity and reliability, the researchers say.
Once its task is complete, the tiny pill-like device would be disposed of through the usual elimination route without harm to the stomach or intestine. This approach avoids the uncomfortable, risky retrieval of such a device via the oral route.
Occult bleeding is usually first identified in patients who have given a stool sample in which blood is found (the fecal occult blood test), but that test can’t identify the source of such blood — intestine or stomach.
The researchers — Hongying Liu, Panpan Qiao, Xueli Wu, Lan Zhu, Xitian Pi, and Xiaolin Zheng, with the Key Laboratory of Biorheological Science and Technology of Ministry of Education, Bioengineering College — say the device is likely to prove safe to use and less invasive than other endoscopic technology and devices for occult-blood testing.
The researchers now plan to take the patent-pending device to clinical safety testing, and then to patients.
Funding
was provided by the National Natural Science Foundation of China and
the Fundamental Research Funds for the Central Universities.
Full disclosure: I am a consultant to Chongqing University, but I
have no connection to this research. — Amara D. Angelica, Editor, KurzweilAIAbstract of International Journal of Biomedical Engineering and Technology paper
Based on capsule endoscopy and Occult Blood (OB) detection theory, an automatic detection capsule system for Gastric Occult Blood (GOB) was proposed. This system utilised the non-invasive deglutible capsule to automatically identify the GOB information and transmitted the detection result to the external device which could display the detection result and identify the OB status with a particular algorithm. Subjects or doctors could discriminate whether OB existed by observing the external device. This paper designed the automatic detection capsule of GOB and the detecting sensor based on collaurum immune theory. We did in vitro experiments to testify the system and acquired the detecting result image information. Experimental results indicated that the effective detection concentration range of the OB sensor was 6-1800 μg/ml. With simplicity and reliability, this system provides a new idea for the screening of early stage gastric cancer.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington