A new process for producing synthetic gasoline based on carbon nanofibers
December 4, 2013
[+]
A chemical system developed by researchers at the University of Illinois at Chicago
can efficiently perform the first step in the process of creating
synthetic gasoline (syngas) and other energy-rich products out of carbon
dioxide.
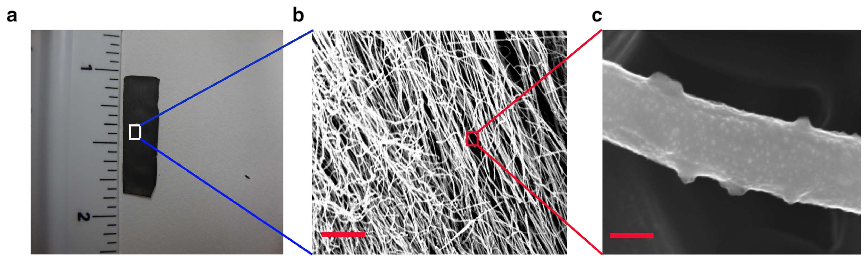
Digital
image of carbon nanofiber mat used as catalyst, (b) SEM image of the
CNF mat displaying entangled fibers (scale bar, 5 mm), (c)
high-resolution SEM image on individual fibers (scale bar, 200 nm).
Random corrugations are visible at the fiber surface. (Credit: Bijandra
Kumar et al./Nature Communications)
The key to the new process is a novel “co-catalyst” system using inexpensive, easy-to-fabricate carbon-based nanofiber materials that efficiently convert carbon dioxide to carbon monoxide, a useful starting material for synthesizing fuels. The findings have been published online in advance of print in the journal Nature Communications.
“I believe this can open a new field for the design of inexpensive and efficient catalytic systems for the many researchers already working with these easily manipulated advanced carbon materials,” says Amin Salehi-Khojin, UIC professor of mechanical and industrial engineering and principal investigator on the study.
Graphitic carbon nanofibers
[+]
Researchers have spent decades trying to find an efficient,
commercially viable first step to chemically “reduce,” or lower the
oxidation state, of carbon dioxide to allow for efficiently synthesizing
gasoline.
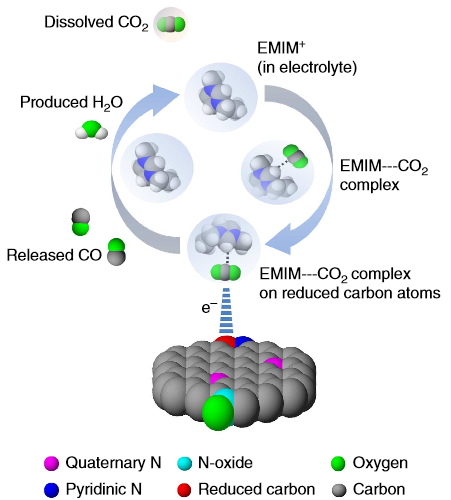
The
CO2 reduction reaction takes place in three steps: (1) an intermediate
(EMIM–CO2 complex) formation, (2) adsorption of EMIM–CO2 complex on the
reduced carbon atoms and (3) CO formation. (Credit: Bijandra Kumar et
al./Nature Communications)
Reducing carbon dioxide is a two-step process, but chemists had commonly used only a single catalyst, Salehi-Khojin said. So he and his colleagues experimented with using different catalysts for each step.
In previous work, Salehi-Khojin used an ionic liquid to catalyze the first step of the reaction, and silver for the final reduction to carbon monoxide. The co-catalyst system was more efficient than single-catalyst carbon dioxide reduction systems, he said.
But silver is expensive. So he and his coworkers set out to see if a relatively new class of metal-free catalysts — graphitic carbon structures doped with other reactive atoms — might work in place of the silver.
They tried a common structural material, carbon nanofiber, which was doped with nitrogen, as a substitute for silver to catalyze the second step.
When these carbon materials are used as catalysts, the doping atoms, most often nitrogen, drive the reduction reaction. But, through careful study of this particular reaction, the researchers found that it was not the nitrogen that was the catalyst; it was the carbon atom.
Bijandra Kumar, UIC research scholar and the other first-author of the paper, said the team “uncovered the hidden mechanism” of the co-catalyzed reaction, which has “opened up a lot of options for designing inexpensive and efficient catalyst system for carbon dioxide conversion.”
Graphene nano-sheets
[+]
“Further, one can imagine that using atomically-thin,
two-dimensional* graphene nano-sheets — which have extremely high
surface area and can easily be designed with dopant atoms like nitrogen —
we can develop even far more efficient catalyst systems,” Kumar said.
Graphene is an atomic-scale honeycomb lattice made of carbon atoms (credit: Wikimedia Commons)
(Examples of doping of graphene are shown here on KurzweilAI.)
“If the reaction happened on the dopant, we would not have much freedom in terms of structure,” said Salehi-Khojin. In that case, little could be done to increase the efficiency or stability of the reaction.
But with the reaction happening on the carbon, “we have enormous freedom” to use these very advanced carbon materials to optimize the reaction, he said.
The researchers hope that their research leads to commercially viable processes for the production of syngas — and even gasoline — from carbon dioxide.
* Would a 3D version of graphene also be useful to explore, as in this use as a catalyst in solar cells?
Abstract of Nature Communications paper
The development of an efficient catalyst system for the electrochemical reduction of carbon dioxideinto energy-rich products is a major research topic. Here we report the catalytic ability of polyacrylonitrile-based heteroatomic carbon nanofibres for carbon dioxide reduction into carbon monoxide, via a metal-free, renewable and cost-effective route. The carbon nanofibre catalyst exhibits negligible overpotential (0.17 V) for carbon dioxide reduction and more than an order of magnitude higher current density compared with the silver catalyst under similar experimental conditions. The carbon dioxide reduction ability of carbon nanofibres is attributed to the reduced carbons rather than to electronegative nitrogen atoms. The superior performance is credited to the nanofibrillar structure and high binding energy of key intermediates to the carbon nanofibre surfaces. The finding may lead to a new generation of metal-free and non-precious catalysts with much greater efficiency than the existing noble metal catalysts.
References:
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington