A DNA nanocage for transporting drugs in the body
December 4, 2013
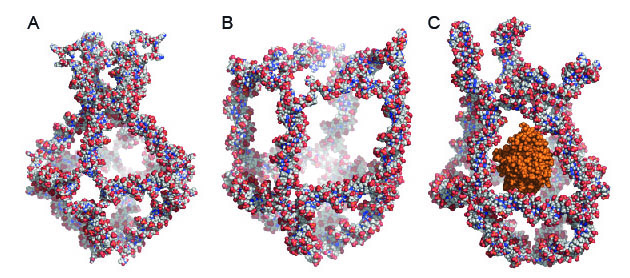
Atomistic
models of a nanocage in which eight unique DNA molecules are mixed
together. The nanocage has four functional elements that transform
themselves in response to changes in the surrounding temperature. These
transformations either close (A) or open (B) the nanocage. By exploiting
temperature changes in the surroundings, the researchers trapped an
active enzyme called horseradish peroxidase (HRP) in the nanocage (C).
(Credit: Sissel Juul)
[+]
Using DNA self-assembly, the researchers designed eight unique DNA
molecules from the body’s own natural DNA molecules. When these DNA
molecules are mixed together, they spontaneously aggregate in a usable
form — a nanocage (a truncated octahedron or eight-sided structure, in
this case).
Cage x-ray images (credit: Sissel Juul et al./ACS Nano)
The nanocage has four functional elements that transform themselves in response to changes in the surrounding temperature. These temperature transformations either close (at 4 degrees C) or open (at 37 degrees C) the nanocage (see figure above).
How to trap a drug
By exploiting the temperature changes in the surrounding environment, the researchers trapped an active enzyme called horseradish peroxidase (HRP) in the nanocage. (They used HRP as a model because its activity is easy to trace.)
The researchers also show that HRP retains its enzyme activity inside the nanocage and converts substrate molecules that are small enough to penetrate the nanocage to new molecular products inside.
The encapsulation of HRP in the nanocage is reversible — the nanocage can release the HRP — or a drug — in reaction to reversed temperature changes. The researchers also show that the DNA nanocage — with its enzyme load — can be taken up by cells in culture.
The researchers expect that this process can be used for delivery to enzyme drugs to targeted diseased cells in the body. (Note the temperature-controlled cargo release presented in the current study is not suitable for in vivo applications.)
“This is the first demonstration of a DNA nanocage that can encapsulate and release a biomolecule without addition of external factors or degradation of the nanocage and by controlling the ambient temperature,” postdoctoral fellow Sissel Juul, Department of Biomedical Engineering, Duke University and an author of the paper, explained to KurzweilAI.
“Also, this nanocage is very small (15–20 nm in outer diameter), so the cavity is the optimal size for most biomolecules such as enzymes. Other 3D DNA nanostructures are bigger and therefore have bigger holes where the cargo can be release from prematurely. By ‘hiding’ a drug or biomolecule inside the DNA nanocage, which is made of natural materials, we can deliver that biomolecule to a diseased cell without the biomolecule being degraded in the process since it is not recognized by the cells. Also, the DNA nanocage itself is very stable because it has no free DNA ends (it is all covalently closed). Free DNA ends trigger the degradation machinery.
“This work should be seen as proof-of-concept that it can be done. We will ourselves work on optimizing for commercial use, but we also hope that others will pick up the principles that we demonstrated and push this technology to the limit.”
The research was carried out at the Department of Molecular Biology and Genetics and the Interdisciplinary Nanoscience Centre (iNANO), Aarhus University, in collaboration with researchers from Duke University (U.S.) and the University of Rome (Italy).
Abstract of ACS Nano paper
We demonstrate temperature-controlled encapsulation and release of the enzyme horseradish peroxidase using a preassembled and covalently closed three-dimensional DNA cage structure as a controllable encapsulation device. The utilized cage structure was covalently closed and composed of 12 double-stranded B-DNA helices that constituted the edges of the structure. The double stranded helices were interrupted by short single-stranded thymidine linkers constituting the cage corners except for one, which was composed by four 32 nucleotide long stretches of DNA with a sequence that allowed them to fold into hairpin structures. As demonstrated by gel-electrophoretic and fluorophore-quenching experiments this design imposed a temperature-controlled conformational transition capability to the structure, which allowed entrance or release of an enzyme cargo at 37 °C while ensuring retainment of the cargo in the central cavity of the cage at 4 °C. The entrapped enzyme was catalytically active inside the DNA cage and was able to convert substrate molecules penetrating the apertures in the DNA lattice that surrounded the central cavity of the cage.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington