On-demand vaccines possible with engineered nanoparticles
January 9, 2014
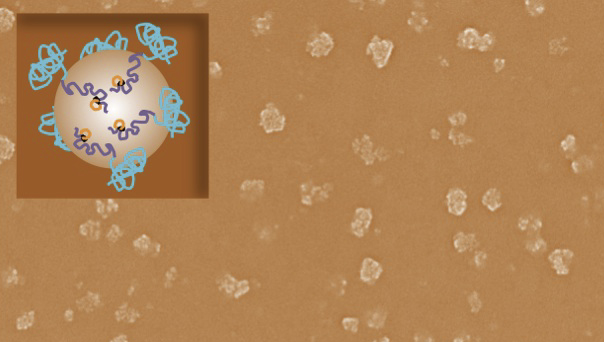
This
image shows a collection of vaccinating nanoparticles from 40 to 50
nanometers in size. The inset graphic is a representation of how the
engineered proteins decorate a nanoparticle’s surface. (Credit:
University of Washington)
Vaccines usually are made en masse in centralized locations far removed from where they will be used. They are expensive to ship and keep refrigerated and they tend to have short shelf lives.
Just-in-time manufacturing
The new technology makes it possible to produce a vaccine on the spot. “For instance, a field doctor could see the beginnings of an epidemic, make vaccine doses right away, and blanket vaccinate the entire population in the affected area to prevent the spread of an epidemic,” said François Baneyx, a UW professor of chemical engineering and lead author of a recent paper published online in the journal Nanomedicine.
In typical vaccines, weakened pathogens or proteins found on the surface of microbes and viruses are injected into the body along with compounds called adjuvants to prepare a person’s immune system to fight a particular disease. But standard formulations don’t always work, and the field is seeking ways to manufacture vaccines quicker, cheaper and tailored to specific infectious agents, Baneyx said.
Vaccinating nanoparticles
The UW team injected mice with nanoparticles synthesized using an engineered protein that both mimics the effect of an infection and binds to calcium phosphate, the inorganic compound found in teeth and bones. After eight months, mice that contracted the disease made threefold the number of protective “killer” T-cells — a sign of a long-lasting immune response — compared with mice that had received the protein but no calcium phosphate nanoparticles.
The nanoparticles appear to work because their 40–50 nm size allows for ferrying the protein to the lymph nodes, where they have a higher chance of meeting dendritic cells, a type of immune cell that is scarce in the skin and muscles, but plays a key role in activating strong immune responses.
In a real-life scenario, genetically engineered proteins based on those displayed at the surface of pathogens would be freeze-dried or dehydrated and mixed with water, calcium and phosphate to make the nanoparticles. This should work with many different diseases and be especially useful for viral infections that are hard to vaccinate against, Baneyx said.
He cautioned, however, that the new technology has only been proven in mice. “It still has to be validated in humans and approved by the FDA, which takes much money and many years,” Baneyx explained to KurzweilAI.
“The approach could be useful in the future for vaccinating people in developing countries, especially when lead time and resources are scarce,” he added. “It would cut costs by not having to rely on refrigeration, and vaccines could be produced with rudimentary equipment in more precise, targeted numbers. The vaccines could be manufactured and delivered using a disposable patch, like a bandage, which could one day lessen the use of trained personnel and hypodermic needles.”
The research was funded by a Grand Challenges Explorations grant from the Bill & Melinda Gates Foundation and the National Institutes of Health.
Abstract of the Nanomedicine: Nanotechnology, Biology and Medicine paper
Distributed and on-demand vaccine production could be game-changing for infectious disease treatment in the developing world by providing new therapeutic opportunities and breaking the refrigeration “cold chain”. Here, we show that a fusion protein between a calcium phosphate binding domain and the model antigen ovalbumin can mineralize a biocompatible adjuvant in a single step. The resulting 50
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington