Extending future electric-car battery life, range
January 12, 2014
[+]
A hybrid anode developed at the Department of Energy’s Pacific Northwest National Laboratory (PNNL) could quadruple the life of the lithium-sulfur batteries proposed for use in electric vehicles.
A
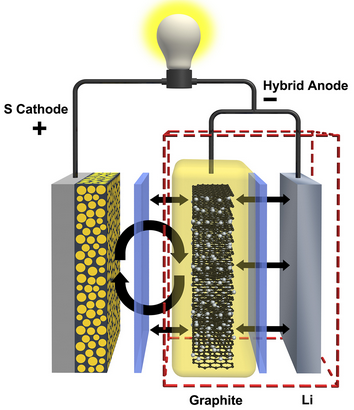
hybrid anode made of graphite and lithium could quadruple the lifespan
of lithium-sulfur batteries (credit: Huang et al, Nature Communications)
“Lithium-sulfur batteries could one day help us take electric cars on longer drives and store renewable wind energy more cheaply, but some technical challenges have to be overcome first,” said PNNL Laboratory Fellow Jun Liu. “PNNL’s new anode design is helping bringing us closer to that day.”
Today’s electric vehicles are commonly powered by rechargeable lithium-ion batteries, which are also being used to store renewable energy. (Also see Organic flow battery promises breakthrough for renewable energy for another storage solution.) But the chemistry of lithium-ion batteries limits how much energy they can store.
Lithium-sulfur batteries could extend range
One promising solution is the lithium-sulfur battery, which can hold as much as four times more energy per mass than lithium-ion batteries. This would enable electric vehicles to drive longer on a single charge and help store more renewable energy. The down side of lithium-sulfur batteries, however, is they have a much shorter lifespan because they can’t be charged as many times as lithium-ion batteries.
The lithium-sulfur battery’s main obstacles are unwanted side reactions that cut the battery’s life short. The undesirable action starts on the battery’s sulfur-containing cathode*, which slowly disintegrates and forms molecules called polysulfides that dissolve into the battery’s electrolyte liquid. The dissolved sulfur eventually develops into a thin film called the solid-state electrolyte interface layer. The film forms on the surface of the lithium-containing anode, growing until the battery is inoperable.
Most lithium-sulfur battery research to date has centered on stopping sulfur leakage from the cathode. But PNNL researchers determined stopping that leakage can be particularly challenging. Besides, recent research has shown a battery with a dissolved cathode can still work. So the PNNL team focused on the battery’s other side by adding a protective shield to the anode.
Quadrupling battery lifespan
The new shield is made of graphite, a thin matrix of connected carbon molecules that is already used in lithium-ion battery anodes. In a lithium-sulfur battery, PNNL’s graphite shield moves the sulfur side reactions away from the anode’s lithium surface, preventing it from growing the debilitating interference layer. Combining graphite from lithium-ion batteries with lithium from conventional lithium-sulfur batteries, the researchers dubbed their new anode a hybrid of the two.
The new anode quadrupled the lifespan of the lithium-sulfur battery system the PNNL team tested. When equipped with a conventional anode, the battery stopped working after about 100 charge-and-discharge cycles. But the system worked well past 400 cycles when it used PNNL’s hybrid anode and was tested under the same conditions.
“Sulfur is still dissolved in a lithium-sulfur battery that uses our hybrid anode, but that doesn’t really matter,” Liu said. “Tests showed a battery with a hybrid anode can successfully be charged repeatedly at a high rate for more 400 cycles, and with just an 11-percent decrease in the battery’s energy storage capacity.”
This and most other lithium-sulfur battery research is conducted with small, thin-film versions of the battery that are ideal for lab tests. Larger, thicker batteries would be needed to power electric cars and store renewable energy. Liu noted tests with a larger battery system would better evaluate the performance of PNNL’s new hybrid anode for real-world applications.
This study was primarily supported by the Department of Energy’s Office of Science (BES), with additional support from DOE’s Advanced Research Projects Agency-Energy, and DOE’s Office of Energy Efficiency and Renewable Energy. Some of this research was performed at EMSL, DOE’s Environmental and Molecular Sciences Laboratory at PNNL.
* Most batteries have two electrodes: one is positively charged and called a cathode, while the second is negative and called an anode. Electricity is generated when electrons flow through a wire that connects the two. Meanwhile, charged molecules called ions shuffle from one electrode to the other through another path: the electrolyte solution in which the electrodes sit.
Abstract of Nature Communications paper
Lithium-sulphur batteries have high theoretical energy density and potentially low cost, but significant challenges such as severe capacity degradation prevent its widespread adoption. Here we report a new design of lithium–sulphur battery using electrically connected graphite and lithium metal as a hybrid anode to control undesirable surface reactions on lithium. Lithiated graphite placed in front of the lithium metal functions as an artificial, self-regulated solid electrolyte interface layer to actively control the electrochemical reactions and minimize the deleterious side reactions, leading to significant performance improvements. Lithium–sulphur cells incorporating this hybrid anodes deliver capacities of >800 mAh g−1 for 400 cycles at a high rate of 1,737 mA g−1, with only 11% capacity fade and a Coulombic efficiency >99%. This simple hybrid concept may also provide scientific strategies for protecting metal anodes in other energy-storage devices.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington